The Mexican pharmaceutical company awarded a contract for two complete high-containment lines for tablet production in August 2018, and Excellence United is currently implementing this joint project. The lines each incorporate a tablet press from Fette Compacting, a granulation and coating unit from Glatt, and – to package the highly toxic solid dose products – a Blister line BEC 300 plus an Uhlmann Labeling Platform ULP. The Uhlmann specialists fitted each BEC 300 blister machine with a customized containment system.
Whether it is just for packaging or, as in this case, involves an integrated line for the production of the solids covering HAPI raw material dosage, tablet production, and packaging, the key criterion of every containment solution is protection: reliable protection of the operator against the active ingredients, and equally reliable protection of the product against contamination from outside or caused by other substances.
Simulate, optimize, and engineer. To ensure accurate customization of the separate line components from Glatt, Fette Compacting, and Uhlmann in accordance with the specific requirements, all the process steps were first jointly defined with the customer. A kick-off meeting was also held in Mexico to clarify the Uhlmann share of the project. Various videos demonstrating setup, cleaning, and troubleshooting procedures were presented.
According to Uhlmann project manager Emanuel Wegis, this approach is “good practice where containment solutions are concerned. Using practical examples, we are able to accurately analyze the individual specifications beforehand. Furthermore, we always carry out a risk assessment. In addition to taking operator protection into account, we also consider production-relevant factors such as batch sizes, materials, and many other aspects.”

By cooperating with Excellence United, our long-term customer has a competent partner for the entire process from production to packaging on high-containment lines – one-stop supply and services.
Andrés Garcia, Managing Director Fette Compacting Mexico, and Uhlmann representative in Mexico

Protection, efficiency, and flexibility. The outcome of this analysis was that the line for the packaging of oncological products must meet the requirements of the second highest protection level OEB5 for highly toxic products. One way of illustrating this is to extrapolate the toxic exposure to the volume of the Empire State Building: no more than one twentieth of a teaspoon of the toxic substance is permitted in the whole building. In addition to these stringent stipulations regarding operator protection, flexibility is a key factor. The line is to seal tablets in film blisters, and package them in cartons – in six different configurations.
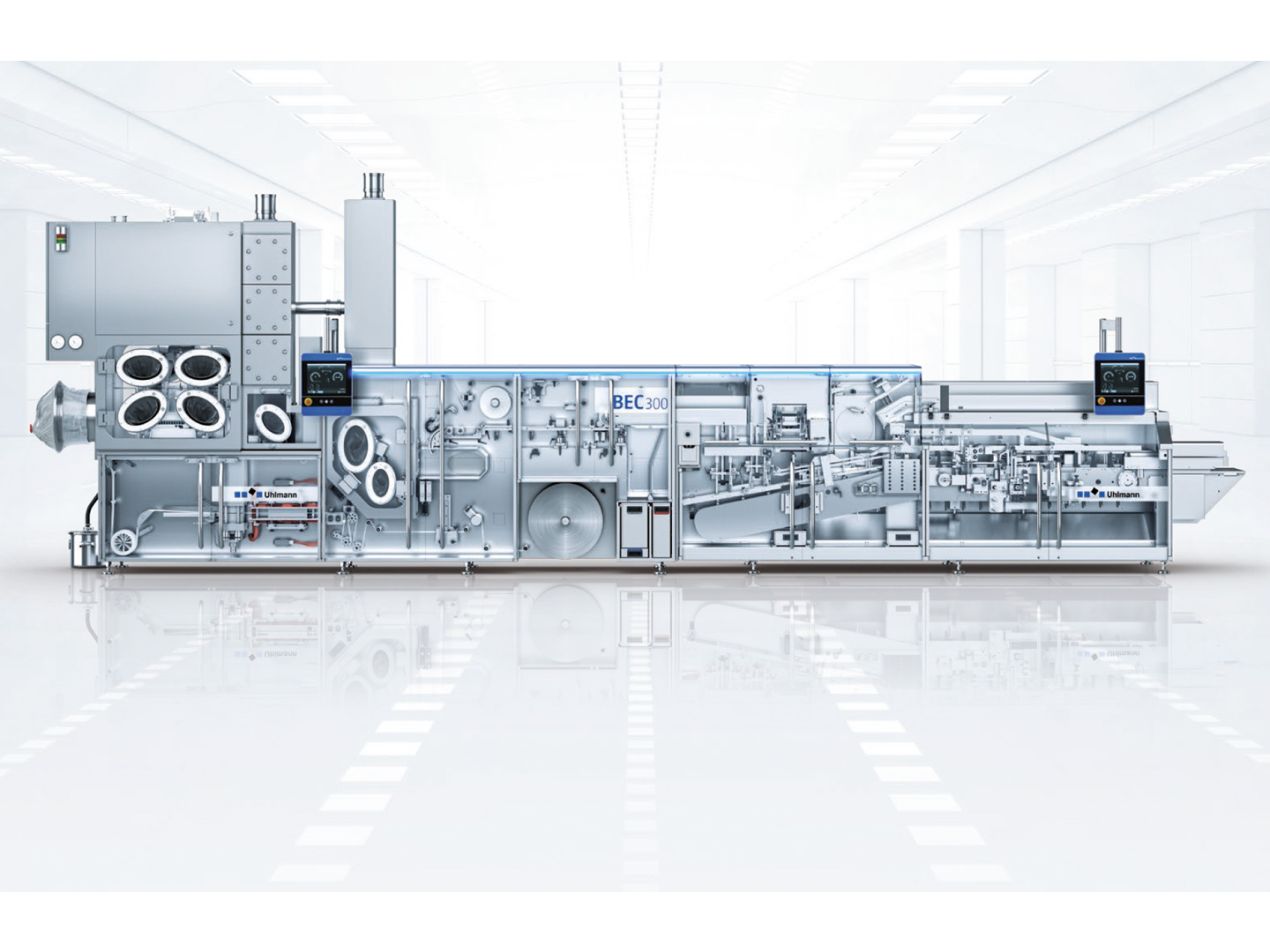
Flexible combination: Blister line BEC 300. Consequently, the Uhlmann specialists recommended a Blister line BEC 300 as the basis for the container lines. Well-proven in many practical applications and offering a stable process, the BEC 300 is ideal for the variable processing of standard-shaped solid dose products. Common forming materials are used to package small to medium-sized batches of up to 70,000 packs. In addition, a number of BEC 300 blister machines have already been upgraded to customized containment solutions.
Negative and positive pressure. The customized pressure zone and climate control concept, based on an air management unit, is a central element of containment. Positive air pressure protects the products against cross-contamination when a product changeover takes places. Negative air pressure prevails at the positions where the operator engages in the process, and where the most dust occurs – usually in the area of the fill section. In addition, there are specific extraction points for this dust, such as at the tablet feeder. Filters for supplied and extracted air are also an integral part of a containment system. The supply air filters ensure the required air purity, while the extraction air filters protect the outside area from contamination. A cooling circuit maintains a constant temperature during the packaging process. Sensors continually measure the temperature as well as the humidity.

"Every containment solution is customized. The specifications always vary and are always very complex. A lot of know-how is needed – and that can only be built up through many successfully implemented projects."
Emanuel Wegis, Project Manager Uhlmann Customized Packaging Systems

Safe zone between feeding and transfer to the cartoner. The solid dose products formed by the Glatt granulator and the Fette Compacting tablet press are forwarded to the line in tablet containers. A lift raises these to the hopper of the feeder. A passive flap in the container base and an active flap in the hopper opening – each from Glatt – engage and tightly link the two elements. The flaps open, allowing the products to flow into the hopper – an airtight process without any operator contact. The flaps then close and the lift removes the empty container.
Double protection also prevails during format changeovers and replacement of the air filters. The operators work in a negative pressure zone and all intervention is carried out through ports with integrated gloves.
Product feeding takes place in a cRABS (closed restricted-access barrier system) zone. This area of the blister machine is enclosed, forming the greatest possible barrier to outside. A containment SimTap places the tablets individually in the blister pockets to keep dust to a minimum. Sealing of the blisters also takes place in the cRABS zone, likewise inspection of the products. Only the camera is positioned outside the zone to simplify cleaning. Faulty blisters are removed via a special magazine, from which the operator can also access IPC blisters.
The last step of this tailored solution is product transfer to the cartoner using a conveyor. Subsequent handling continues “normally”: carton printing, filling, checkweighing, and the application of tamperevident labels using the Uhlmann Labeling Platform.
Issue 03/2020: Person- and product-protectors



![[Translate to 中国人:] [Translate to 中国人:]](/fileadmin/Redakteure_Mainpage/01_Products/03_Blister_lines/01_BEC_300/01_ANSICHT_Blisterlinien_BEC300.jpg)
![[Translate to 中国人:] [Translate to 中国人:]](/fileadmin/Redakteure_Mainpage/01_Products/07_Competences/01_Containment/01_ANSICHT_Kompetenzen_Containment.jpg)
![[Translate to 中国人:] [Translate to 中国人:]](/fileadmin/Redakteure_Mainpage/01_Products/07_Competences/02_Customized_solutions/UHL-18-011-Headerbild-Kundenloesungen.jpg)