The future of parenteral packaging is flexible
In an ever-changing world, staying flexible is a must. The parenteral market is experiencing unprecedented growth and transformation, with new product formats, delivery systems, and regulatory requirements emerging constantly. Uhlmann empowers pharmaceutical companies to meet any challenge in parenteral packaging, offering unmatched flexibility in handling new products, special formats, diverse materials, and any batch size – so that you are always ready for the future.
Stay flexible – your competitive advantage
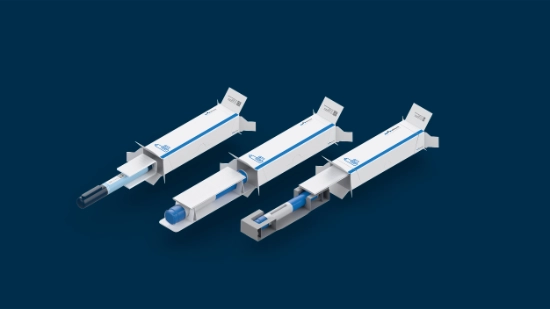
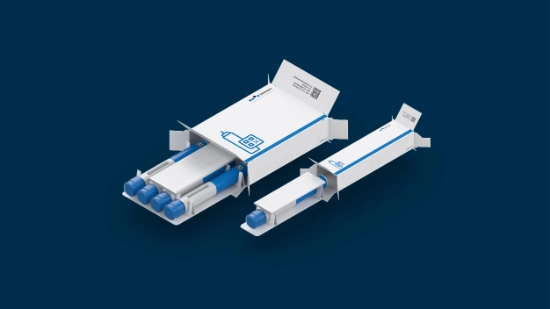
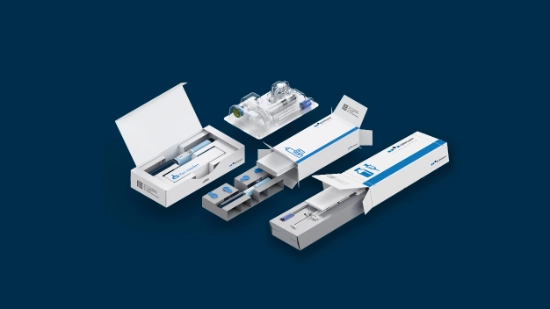
From cartridges in blisters to autoinjectors in paper trays, from stackable to slim trays, from small to big batches: Parenteral packaging demands adaptability across multiple dimensions.
Uhlmann is your long-term partner for flexible parenteral packaging, providing machines, services, and digital solutions that adapt to whatever you need to pack next.
The three dimensions of flexibility
- MaterialKey to fulfilling product requirements and reaching sustainability goals.
- ProductAccommodates diverse forms, ranging from pre-filled syringes to autoinjectors.
- FormatEnables manufacturers to adapt to different space and logistical requirements.
Your key advantages from working with Uhlmann
 Customized packaging solutionsReduce costs, improve sustainability, and enable more efficient logistics – with solutions like slim trays that are tailored to your product’s unique requirements.
Customized packaging solutionsReduce costs, improve sustainability, and enable more efficient logistics – with solutions like slim trays that are tailored to your product’s unique requirements. High performance technologyStreamline your packaging processes with smart automation solutions like Pexcite, and with technology that enables fast yet gentle processing.
High performance technologyStreamline your packaging processes with smart automation solutions like Pexcite, and with technology that enables fast yet gentle processing. Packaging competenceOur seasoned professionals combine process optimization, material excellence, and engineering expertise to create the optimum solution for your packaging challenge.
Packaging competenceOur seasoned professionals combine process optimization, material excellence, and engineering expertise to create the optimum solution for your packaging challenge.
Custom solutions that enhance your success
Discover packaging tailored to your product’s needs. We optimize materials and formats for configurations built to your exact dimensions, ensuring seamless integration with your supply chain through collaborative development. The result: innovative solutions like our slim cardboard trays that use less material, lowering your costs and environmental impact.
Power to your packaging line
Streamline your packaging processes for optimum performance. Our systems provide high-speed throughput, while innovative feeders ensure gentle, damage-free handling. You can further automate your entire line with digital solutions like Pexcite to reduce operator workload and eliminate the risk of human error.
From material to machine: Benefit from Uhlmann’s extensive knowledge
Uhlmann’s packaging competence delivers end-to-end solutions tailored to your unique requirements. Covering material choice, format options, and packaging-specific parameters, our specialists collaborate with you from the initial concept right through to final implementation – all while incorporating your existing suppliers, to create optimum solutions that respect your established relationships.

Download our white paper
Whether it’s a medical pen, autoinjector, or vial, new therapy concepts and rising demands call for flexible and future-proof solutions.
Our latest white paper gives you insights into:
- Key developments in the pharmaceutical industry
- The growing need for flexibility in parenteral packaging
- How Uhlmann combines gentle handling with high efficiency
We are here for you
You want to learn more about our packaging solutions for your parenteral products? We are happy to help!

Markus Funk
EuropeTechnical Sales Manager
Christian Mueller
North & South AmericaSales Engineering Manager
Meeno He
AsiaSenior Project Engineering Manager, UCN