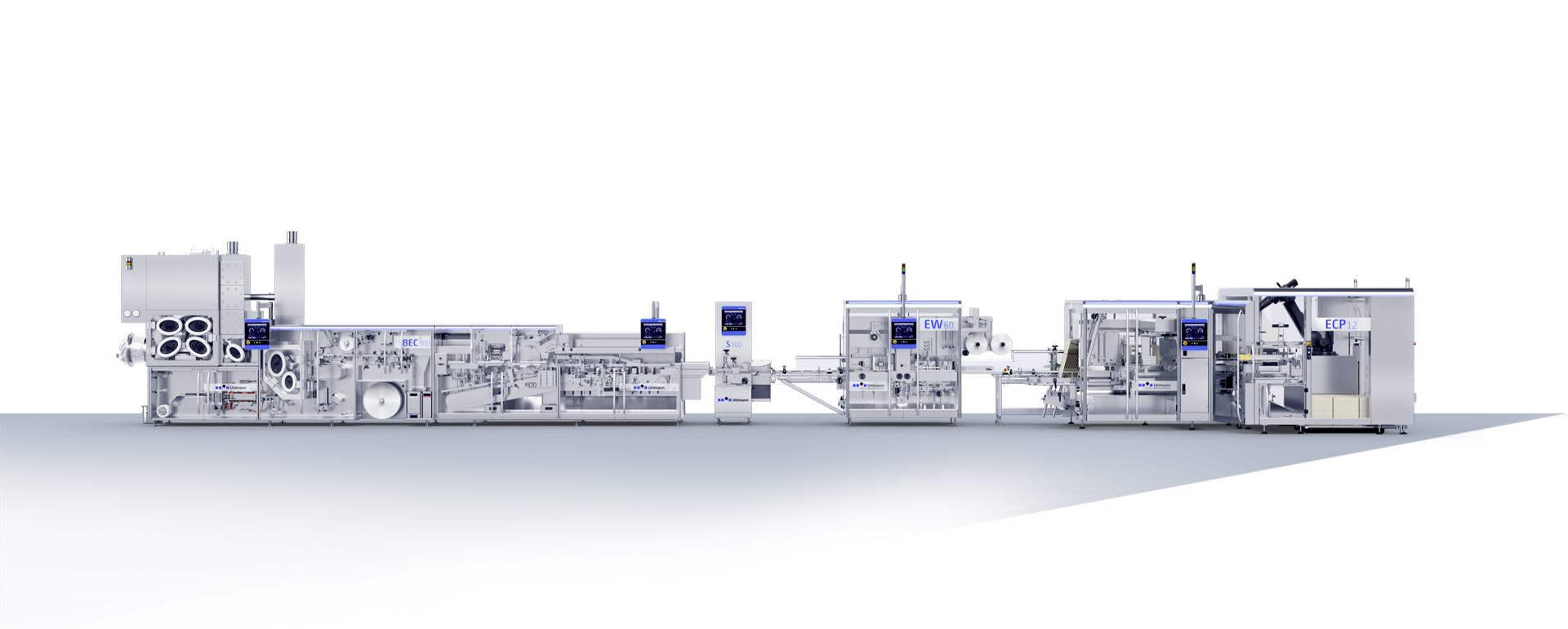
Containment Packaging - individual and efficient
Containment Packaging - individual and efficient
Currently, more than 50 percent of all highly potent solid products are still packaged in the cleanroom. However, the trend is clearly toward containment lines – both worldwide and across all segments. There are several reasons for this; the proportion of highly potent active ingredients continues to grow strongly, thus requiring even better protection for employees, while the official requirements are also increasing. Also, every year, new, highly sensitive products come onto the market whose effectiveness suffers due to environmental influences.
Any containment solution is always about two key factors: reliable protection of operators from the active ingredients, alongside equally reliable protection of the product from contamination from the outside or from other active ingredients.
White paper: Cost-Efficient Containment in Pharmaceutical Packaging

White paper: Cost-Efficient Containment in Pharmaceutical Packaging
Uhlmann has summarized its expertise on the subject of containment in the white paper “Cost-Efficient Containment in Pharmaceutical Packaging”. The packaging specialists describe how containment measures can be effectively integrated into a packaging line, explaining how time and costs can be reduced as a result.
Advantages of the Containment at a glance
Operators work without special protective suits and with regular breaks
No time-consuming entry and exit of personnel and material and significantly reduced effort for format change and cleaning
Safe exclusion of cross-contamination
Less space required for the line
Faster return on investment especially with frequent product changes
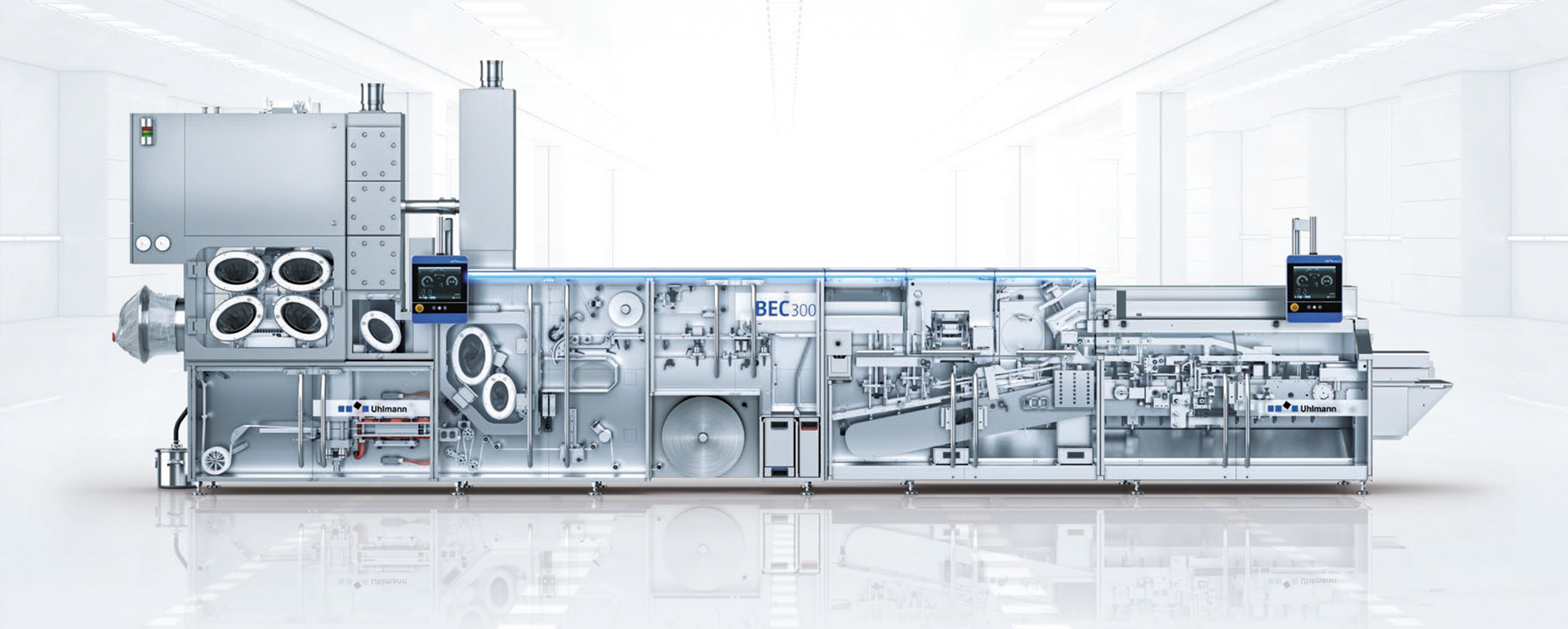
Containment in detail
Inline control for maximum safety throughout the process
Do all the tablets have the correct shape and color, are they all intact? Is each blister pocket correctly filled? Is the imprint on the cover film positionally correct and precise? With Uhlmann inspection systems , you have full control at all times and throughout the process – along with the certainty that only good products are passed on to the next process step. The camera systems are integrated directly at the appropriate positions in the process, and inspection takes place in real time and at full machine speed.
Personal safety
How much dust is developing? What effect does this dust have on the operator? Various tests are used to classify the active substance and product in the OEL pyramid with the various Occupational Exposure Limits. The OELs define the level that the respective containment solution must have – from OEB 1 Non-toxic to 6 Extremely toxic.
Product protection
Sensitive products must be protected from humidity, temperature fluctuations, UV radiation and contamination until the blister is securely sealed. In many cases, a containment concept is only required in the direction of the operator or product. However, if drugs are highly active and highly sensitive, double containment is required. Here, too, Uhlmann's experts have the necessary experience to safely implement such a demanding project. In addition, with the Excellence United companies, partners are available to also integrate upstream processes such as pressing and tableting of the products into an integrated concept.
Provisioning and feeding of the products
To protect your operators from the active ingredients and your products from contamination, the solid products arrive at the packaging line in a closed container. In the example, a drum with a passive valve docks with the line, which in turn is equipped with an active valve. The outer sides of the two flaps lie against each other and are rotated 90° vertically. As a result, the solid products trickle into the Containment SimTap in an airtight manner and without operator contact - a standard design, but completely enclosed. The feeder inserts the tablets individually into the blister pockets with only a small amount of dust.
Whether it's a format change or cleaning in the infeed unit, everything takes place with operator protection, both with negative pressure and with integrated gloves at the appropriate positions in the process.
Air Management Unit
The central element of each containment packaging line is an individual pressure zone and conditioning concept, tailored to the rooms and product requirements. It is implemented with the help of an Air Management Unit, with:
- Overpressure to protect products from cross-contamination during product changeovers
- Negative pressure to protect operators wherever they intervene in the process and where the most dust is generated – usually in the filling section area
- Targeted extraction points for this dust, e.g. at the tablet feeder
- Conditioning of the supplied air with regard to temperature, humidity and purity
High precision die cutting for intact blisters
To ensure maximum product and personal protection until the blister is opened, it is die-cut with the highest precision. This is the only way to ensure that the blister pockets remain intact, and that neither active ingredients can escape to the outside nor environmental influences can enter the blister. Laser pocket control in the die cutter ensures absolutely accurate blister dimensions, while lightweight tools reduce changeover times.
IPC Control: Automatically and at any time
The operator can request the desired number of blisters at any time via the HMI. The blisters are automatically discharged from the process into a special chute and can be removed at an ergonomic height.